-
Optical Tomography
People often automatically connect "optical imaging" with beautiful and colorful microscopic images of cells. It is true that traditional and emerging microscopy techniques (con-focal, 2-photon, photoacoustic microscopy, OCT etc) are important members of optical imaging, but there are more.
The COTI lab focuses on a particular type of optical imaging, called diffuse optical tomography (DOT). A unique capability of DOT is that it can "see through" several or even over a dozen centimetres of opaque tissues without needing to cut open the tissue (also known as non-invasiveness), kind of like x-ray CT or MRI. However, compared to x-ray CT, DOT only uses safe low-energy near-infrared photons, similar to the sunlight shining on your face when you take an outdoor walk. In comparison, x-ray photons are much more energetic and may damage cells if the radiation is prolonged. Using DOT, we can computationally reconstruct 3-D maps of blood concentrations (how much blood per volume) and oxygen saturation (the percentage of red blood cells that carry oxygen) in a thick tissue sample. These physiological information are excellent indicators for diseases such as the presence of malignant tumors, inflammation or vascular diseases. In comparison, most microscopy techniques can only see through tissues no deeper than 1 mm.
In our research, we primarily use red and infrared wavelengths in the light spectrum to perform DOT measurements. This is because the major light absorbers in the tissue, known as chromophores, are primarily oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (HbR), water (H2O), lipids, melanin (the pigment made up our skin and hair colors) etc, have a relatively low absorption between 600 nm (red-colored) and 1000 nm (infrared and invisible) in light wavelengths. This window is referred to as the (1st) optical window. The provides researchers an opportunity to image deeply embedded tissue structures using red and near-infrared light.
Although it sounds fantastic if one can use light to create tomographic (i.e. 3-D) images of tissues, DOT is actually pretty difficult to do in general. There are a lot of challenges that have to be solved first in order to make this work. First, near-infrared photons are much much less energetic than x-ray photons. So, when it enters the tissue, it does not follow a straight-line path like x-ray does (after collimation of course), instead, it is bounced around, a lot, by tissue cells like a ping-pong ball. This is called scattering. Tissue scattering is the biggest "enemy" for creating clear images (for any modality, not just DOT), because people can not precisely know where such photon had traveled.
Photons not only scatter a lot in the tissue, they also attenuate quickly by hitting absorbers like blood. The high absorption of tissues makes light signals extremely weak (10-7~10-8) after propagating centers of tissues and arriving at a detector. So, in order to get enough photons to give meaningful measurements, DOT requires to use extremely sensitive light detectors, such as PMT (photon multiplier tubes) and APD (avalanche photodiode), EM-amplified CCD cameras etc.
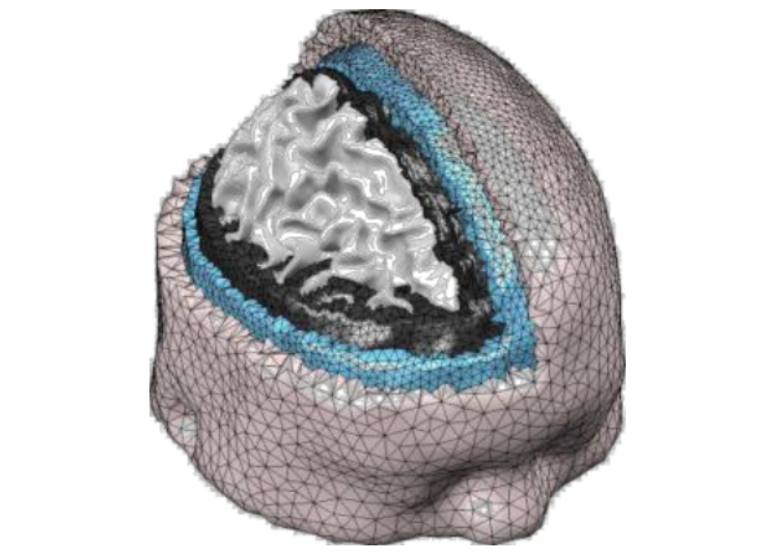
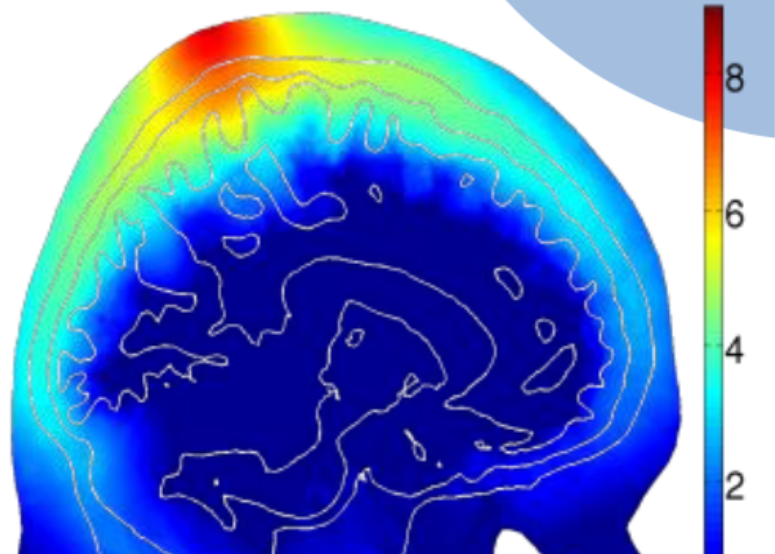
The presence of both scattering and absorption makes light spread-out in the tissue like a drop of food coloring in the water - they spread out in all directions and attenuate exponentially as they expand. The light intensity through complex tissues can be approximated by a partial differential equation known as the diffusion equation (DE). If tissues have complex structures (such as human brain with multiple layers, bones, or skins), one has to solve this DE numerically using computer algorithms. This often requires specialized software and we have to write our own solvers.
Modeling how light travels is only a part of DOT. In real-world applications, the tissue structure is not even known (if it had been known ahead of time, then there was no need to do any imaging). So, how can one figure out the internal tissue structure by shining light on the surface of an unknown tissue structure (like human head or breast), and then measuring light intensity reemerging on the tissue surface is the core question in DOT (and many tomographic imaging modalities in general). This is the so called "inverse problem". In the case of x-ray CT, the inverse problem can be solved by a linear equation, but this no longer works in DOT because of the presence of scattering and complex relationship between absorption/scattering to light distribution. To solve the DOT image reconstruction problem, we have to develop sophisticated reconstruction algorithms, and this is still a major research area for innovation, including how to improve image quality using prior information.
In short, our lab uses near-infrared light to create 3-D maps of tissue blood concentrations and other useful markers to help doctors to find tumors and diseases many centermeters below the tissue surface. We develop both optical imaging instruments as well as sophisticated computer programs to make DOT a promising technology for the future of healthcare.
-
Point-of-Care
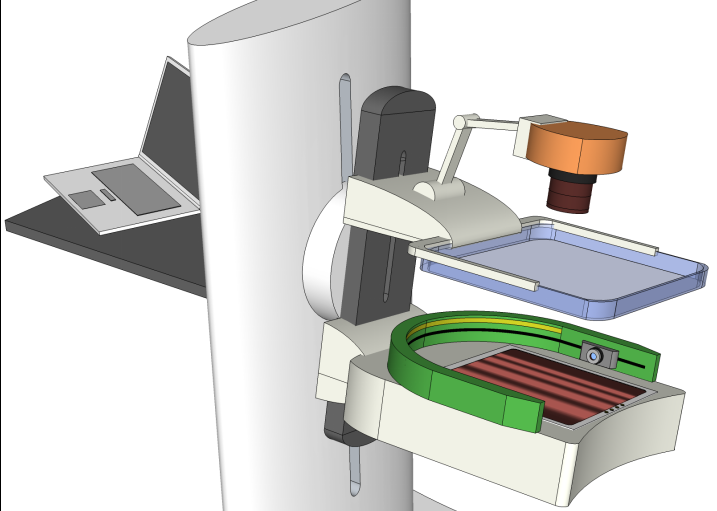
Every year, nearly 3 million newborns die globally within the first 4 weeks of life. In the meantime, over 287,000 women die during pregnancy or following childbirth. Nearly all of these deaths occur in low and middle-income countries (LMICs). We felt the urgency to eliminate this disparity in healthcare and are determined to make innovations to bring life-saving point-of-care diagnostic tools to the resource-poor countries. In 2011, with the funding support from the Bill&Melinda Gates Foundation, we started an investigation on developing low-cost mobile-phone based near-infrared imaging systems. In 2013, we worked with clinical collaborators and initiated another project to develop a mobile-phone thermal imager for diagnosing childhood pneumonia. We hypothesized that the asymmetry in the chest temperature distributions is a marker to the inflammation in the lung due to pneumonia. Our pilot study successfully validated this hypothesis, showing 100% sensitivity and 75% specificity from 12 subjects. The exciting preliminary results from both projects ensured successful funding from the US Agency for International Development (USAID) in the Saving Lives at Birth campaign. In 2015, our project on non-contact mobile oximeter was named one of the 30 leading innovations in the Innovation Countdown 2030 Initiative’s inaugural report (http://ic2030.org/). In 2017, we completed the design of three prototypes of mobile-based oximeters and will begin our clinical studies at MGH startin in the last quarter of 2017. -
Multi-modal Imaging
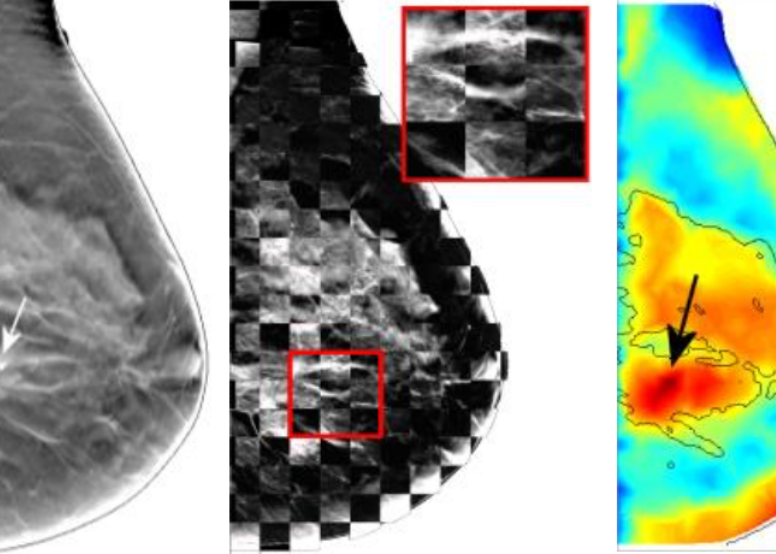
Early diagnosis of breast cancer is critically important. However, the current clinical approach, x-ray mammography, is poor in specificity – over 85% of the recalls yielded benign findings. Furthermore, mammography misses 40% of early stage cancers. Over the past years, we have been developing a novel imaging technique to find breast cancers by combining safe, non-invasive near-infrared diffuse optical imaging with high-resolution x-ray mammography. Since 2009, we've been leading this research and conducted a 470-patient clinical study in collaboration with Dr. Daniel Kopans from MGH Avon Center. With our innovative image reconstruction algorithms, we have demonstrated the potentials in differentiating malignant from benign lesions using the functional and structural information together. Our findings were highlighted as a front-cover article in Radiology. In 2011, Dr. Fang started a new collaboration with Philips Healthcare to accelerate the clinical translation of this technique. Recently, we published a clinical study showing viability of adding functional breast assessment to all existing mammography systems. In 2016, we filed a provisional patent on a highly effective computer-aided detection (CAD) technique to automatically locate malignant tumors using optical data. This was possible using conventional approaches. These innovations, if successfully translated to the clinic, are expected to positively impact breast cancer patient management by significantly enhancing diagnostic accuracy and early detection. This breast imaging project was highlighted by the former Massachusetts Governor Deval Patrick in his speech during the “Friends of Cancer Research” forum in 2014. -
GPU Photon Modeling
Biophotonics techniques play increasingly important roles in drug discovery, disease diagnosis, medical interventions as well as fundamental science research. The success of many biophotonics techniques is critically dependent on the ability to model complex interactions between photons and biological tissues accurately and efficiently. In 2009, Dr. Fang published one of the first papers using graphics processing units (GPUs) to accelerate 3D photon transport simulations. His code demonstrated a revolutionary 300-1000x acceleration and was considered “game-changing” by Dr. Simon Arridge (UCL), a pioneer in optical tomography. In 2010, Dr. Fang published a new mesh-based Monte Carlo algorithm. This paper became the most downloaded paper for Biomedical Optics Express in 5 consecutive months in 2013! So far, these works have attracted over 550 citations, 1,000 registered users and 15,000 downloads worldwide. Our open-source Monte Carlo simulation packages are actively used by many optical imaging research labs across the world. Our registration data also indicates that over 30 global companies utilize our software in their development, including Canon, Sony and Ricoh. In 2015, we were awarded an NIH R01 grant to continue leading this large user community and the development of novel massively parallel computational techniques.
-
 Optical Tomography
Optical Tomography
-
 Translational Research
Translational Research
-
 GPU Photon Modeling
GPU Photon Modeling
-
 Point-of-care
Point-of-care
-
 Multi-modal Imaging
Multi-modal Imaging
![[Home]](upload/lab_full_logo.png)