-
Optical Tomography
People often automatically connect "optical imaging" with beautiful and colorful microscopic images of cells. It is true that traditional and emerging microscopy techniques (con-focal, 2-photon, photoacoustic microscopy, OCT etc) are important members of optical imaging, but there are more.
The COTI lab focuses on a particular type of optical imaging, called diffuse optical tomography (DOT). A unique capability of DOT is that it can "see through" several or even over a dozen centimetres of opaque tissues without needing to cut open the tissue (also known as non-invasiveness), kind of like x-ray CT or MRI. However, compared to x-ray CT, DOT only uses safe low-energy near-infrared photons, similar to the sunlight shining on your face when you take an outdoor walk. In comparison, x-ray photons are much more energetic and may damage cells if the radiation is prolonged. Using DOT, we can computationally reconstruct 3-D maps of blood concentrations (how much blood per volume) and oxygen saturation (the percentage of red blood cells that carry oxygen) in a thick tissue sample. These physiological markers are excellent indicators for diseases such as malignant tumors, inflammation or vascular diseases. In comparison, most microscopy techniques can only see through tissues no deeper than 1 mm.
In our research, we primarily use red and infrared wavelengths in the light spectrum to perform DOT measurements. This is because the major light absorbers in the tissue, known as chromophores, are primarily oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (HbR), water (H2O), lipids, melanin (the pigment made up our skin and hair colors) etc, have a relatively low absorption between 600 nm (red-colored) and 1000 nm (infrared and invisible) in light wavelengths. This window is referred to as the (1st) optical window. The provides researchers an opportunity to image deeply embedded tissue structures using red and near-infrared light.
Although it sounds fantastic if one can use light to create tomographic (i.e. 3-D) images of tissues, DOT is actually pretty difficult to do in general. There are a lot of challenges that have to be solved first in order to make this work. First, near-infrared photons are much much less energetic than x-ray photons. So, when it enters the tissue, it does not follow a straight-line path like x-ray does (after collimation of course), instead, it is bounced around, a lot, by tissue cells like a ping-pong ball. This is called scattering. Tissue scattering is the biggest "enemy" for creating clear images (for any modality, not just DOT), because people can not precisely know where such photon had traveled.
Photons not only scatter a lot in the tissue, they also attenuate quickly by hitting absorbers like blood. The high absorption of tissues makes light signals extremely weak (10-7~10-8) after propagating centers of tissues and arriving at a detector. So, in order to get enough photons to give meaningful measurements, DOT requires to use extremely sensitive light detectors, such as PMT (photon multiplier tubes) and APD (avalanche photodiode), EM-amplified CCD cameras etc.
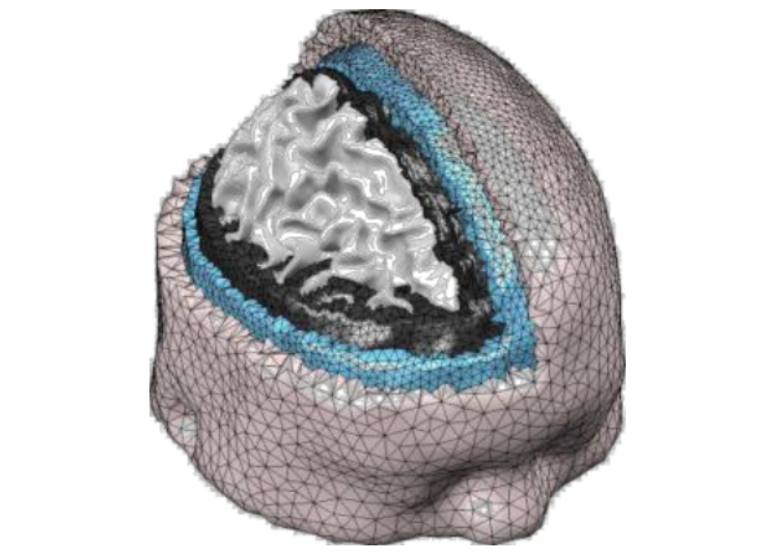
The presence of both scattering and absorption makes light spread-out in the tissue like a drop of food coloring in the water - they spread out in all directions and attenuate exponentially as they expand. The light intensity through complex tissues can be approximated by a partial differential equation known as the diffusion equation (DE). If tissues have complex structures (such as human brain with multiple layers, bones, or skins), one has to solve this DE numerically using computer algorithms. This often requires specialized software and we have to write our own solvers.
Modeling how light travels is only a part of DOT. In real-world applications, the tissue structure is not even known (if it had been known ahead of time, then there was no need to do any imaging). So, how can one figure out the internal tissue structure by shining light on the surface of an unknown tissue structure (like human head or breast), and then measuring light intensity reemerging on the tissue surface is the core question in DOT (and many tomographic imaging modalities in general). This is the so called "inverse problem". In the case of x-ray CT, the inverse problem can be solved by a linear equation, but this no longer works in DOT because of the presence of scattering and complex relationship between absorption/scattering to light distribution. To solve the DOT image reconstruction problem, we have to develop sophisticated reconstruction algorithms, and this is still a major research area for innovation, including how to improve image quality using prior information.
In short, our lab uses near-infrared light to create 3-D maps of tissue blood concentrations and other useful markers to help doctors to find tumors and diseases many centimetres below the tissue surface. We develop both optical imaging instruments as well as sophisticated computer programs to make DOT a promising technology for the future of healthcare.
-
Translational Imaging
The COTI lab is not only interested in exploring new optical imaging techniques and state-of-the-art computational algorithms, it also aims to translate these innovations from bench-side to the bed-side and make real-world impact towards better clinical diagnosis and treatment of diseases. Because of this, we are actively collaborating with clinicians and develop solutions to address particular challenges arose in their applications, and conducting human-based clinical studies to evaluate the efficacy of these devices. Among these efforts, we want to particular highlight the below projects.Multi-modal breast imaging system
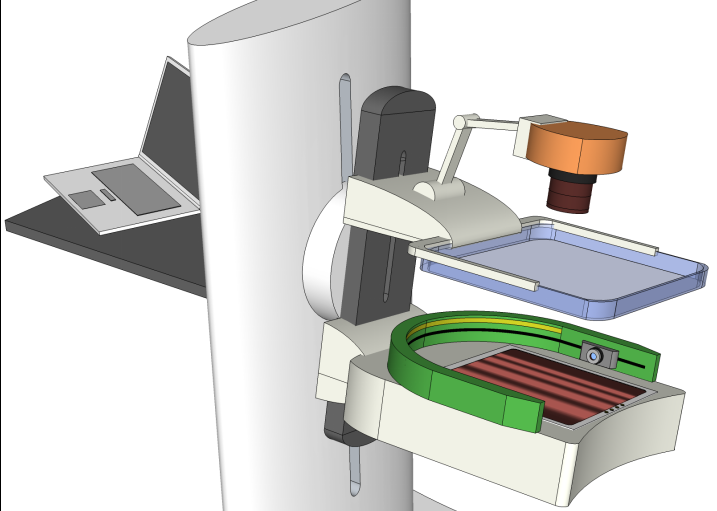
In our lab, we design optical tomography systems that can help diagnose breast cancers. This imaging system utilizes the diffuse optical tomography (DOT) technique and can detect deeply embedded tumors without needing surgery or using ionizing radiation (like x-ray or gamma-ray). A unique aspect of this system is that it can be either mounted on a conventional x-ray mammography system and acquire optical and x-ray images simultaneously (called multi-modal imaging), or acquire optical scans and x-ray scans asynchronously and apply image data fusion in our advanced prior-guided reconstruction algorithm.Wearable Modular fNIRS system
The functional infrared-spectroscopy, or fNIRS, technique is new neuroimaging to detect brain activities, similar to fMRI, EEG (electroencephalography) or MEG (megnetoencephalography), but only using near-infrared light. The fNIRS technique is rapidly getting popular and has seen an exponential grown in publications in the past decade.
Despite that an fNIRS system is significantly more compact and portable compared to fMRI or MEG machines, it remains difficult to be used outside of a lab environment due to the bulky optical-fiber based headgear design and cart-sized optical source/detector units. There is an increasing need in developing wearable fNIRS system that allow clinicians and scientists to monitor brain activities in a more natural environment.
Starting in 2018 with an NIH Brain Initiative R01 award, our team has developed an ultra-compact, wearable, flexible circuit based, modular, wireless, 3-D aware fNIRS system - named MOBI (mobile brain imager). We are currently starting the clinical testing of this device with our collaborators.
Photobiomodulation dosage estimation
Photobiomodulation (PBM) is increasing used in treatment of a wide range of diseases related to inflammation and metabolic disorders, supported by abundant anecdotal and clinical evidences. The administration of this therapy is quite simple - i.e. shining visible and near-infrared light on the skin near the regions of interest, however, its biological mechanism as well as how much light dosage should one use in a subject is not well understood.
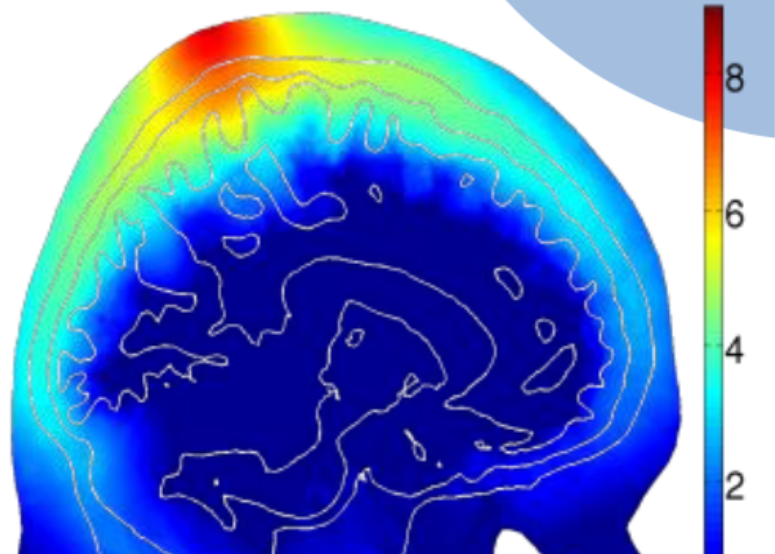
A few years ago, we started a collaboration with Dr. Paolo Cassano at MGH, one of the world's leading experts in PBM research, and used our advanced Monte Carlo light simulation engine and systematically studied the light energy deposition of PBM treatment in the brain. Through this simulation based study, we have gain better understandings on optimal illumination wavelengths, placement positions, optimal treatment time over different ages. This allows clinicians to perform personalized treatment planning as well as optimizing treatment device parameters and placement.
-
Low-cost Point-of-Care Devices
Every year, nearly 3 million newborns die globally within the first 4 weeks of life. In the meantime, over 287,000 women die during pregnancy or following childbirth. Nearly all of these deaths occur in low and middle-income countries (LMICs). We felt the urgency to eliminate this disparity in healthcare and are determined to make innovations to bring life-saving point-of-care diagnostic tools to the resource-poor countries. In 2011, with the funding support from the Bill&Melinda Gates Foundation, we started an investigation on developing low-cost mobile-phone based near-infrared imaging systems. In 2013, we worked with clinical collaborators and initiated another project to develop a mobile-phone thermal imager for diagnosing childhood pneumonia. Our successful initial effort also led to a grant from the US Agency for International Development (USAID) in the Saving Lives at Birth campaign. In 2015, our project on non-contact mobile oximeter was named one of the 30 leading innovations in the Innovation Countdown 2030 Initiative’s inaugural report (http://ic2030.org/). -
Multi-modal Imaging
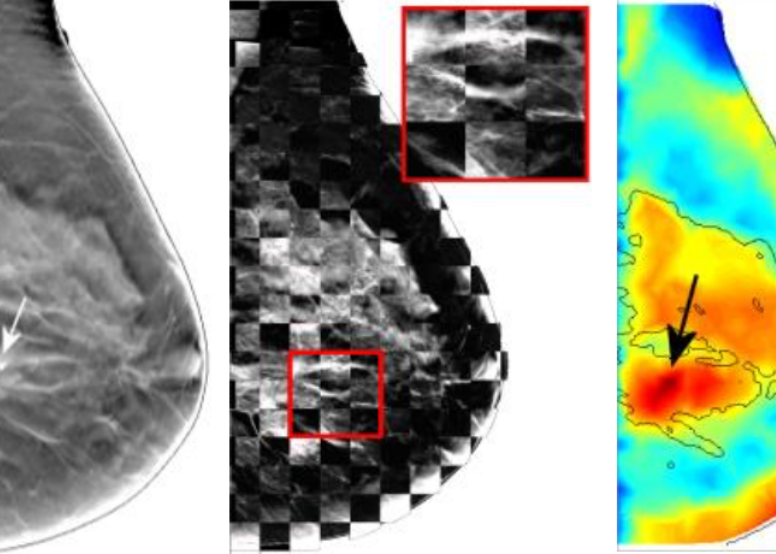
Multi-modal imaging is increasingly used in modern medical imaging. The idea of jointly using multiple imaging modalities, with one focusing on imaging structural details, and another one focusing on tissue functions, has led to the success of PET/CT - a modality that is widely used in clinics today. A similar interest has also been seen in the field of biomedical optics. Our lab has published several works in combining x-ray mammography with DOT to better diagnose breast cancers. In addition to build optical breast imagers that can be used simultaneously or asynchronously with x-ray mammography, we have also investigated heavily advanced image reconstruction algorithms that better "fuse" the structural image with functional measurements like DOT. There are still a lot to explore in terms of enhancing DOT image resolution using multi-modal measurements.In our project of multi-modal breast imaging system, we want to add functional information using DOT to the morphological (tissue shapes) information provided by conventional modalities such as x-ray mammography or tomosynthesis. As you know, early diagnosis of breast cancer is critically important. However, the current clinical approach, x-ray mammography, is poor in specificity – over 85% of the recalls yielded benign findings. Furthermore, mammography misses 40% of early stage cancers. Over the past decade, we have been developing a novel imaging technique to find breast cancers by combining safe, non-invasive near-infrared diffuse optical imaging with high-resolution x-ray mammography. Since 2009, we've been leading this research and conducted a clinical study of hundreds of patients, in collaboration with world's leading-expert in breast imaging, Dr. Daniel Kopans from MGH Avon Center. With our innovative image reconstruction algorithms, we have demonstrated the potentials in differentiating malignant from benign lesions using the functional and structural information together. Our findings were highlighted as a front-cover article in Radiology. In 2011, our lab started a collaboration with Philips Healthcare to accelerate the clinical translation of this technique. Our breast imaging research project was highlighted by the former Massachusetts Governor Deval Patrick in his speech during the “Friends of Cancer Research” forum in 2014.
Currently, we are developing our next-generation breast imaging system, OMCI (optical mammography co-imager), funded by an NIH R01 grant from the National Cancer Institute (NCI).
-
GPU-accelerated Photon Modeling
The success of many emerging biophotonics techniques, including DOT, DCS, PAT and fNIRS, is critically dependent on the ability to model complex interactions between photons and biological tissues accurately and efficiently. In 2009, our lab published the first paper using graphics processing units (GPUs) to accelerate 3-D photon transport simulations in heterogeneous tissues. Our open-source code, Monte Carlo eXtreme or MCX demonstrated a 300-1000x acceleration and has been widely adopted by the biophotonics research community since. In 2010, our lab also published a highly accurate and flexible Monte Carlo light transport simulation method called mesh-based Monte Carlo (or MMC). This paper became the most downloaded paper for Biomedical Optics Express in 5 consecutive months in 2013.Supported by an NIH NIGMS R01 grant, our team have been continuously improving MCX/MMC, and made it one of the fastest and feature-rich light transport simulation platform available today. As of 2020, our papers have attracted over 1100 citations, 2,400 registered users and 30,000 downloads worldwide. In the meantime, we are continue pushing the state-of-the-art of Monte Carlo light modeling and developed dual-grid MMC (DMMC), split-voxel MC (SPMC), implicit MMC (iMMC), photon-replay, photon-sharing etc in the past years.
If you have a strong background in GPU programming, computational physics, and open-source software development, please reach out to Dr. Fang. We are constantly expanding our team to develop top-of-the-line software to our community.
-
 Optical Tomography
Optical Tomography
-
 GPU Photon Modeling
GPU Photon Modeling
-
 Multi-modal Imaging
Multi-modal Imaging
-
 Point-of-care
Point-of-care
-
 Translational Research
Translational Research
![[Home]](upload/lab_full_logo.png)